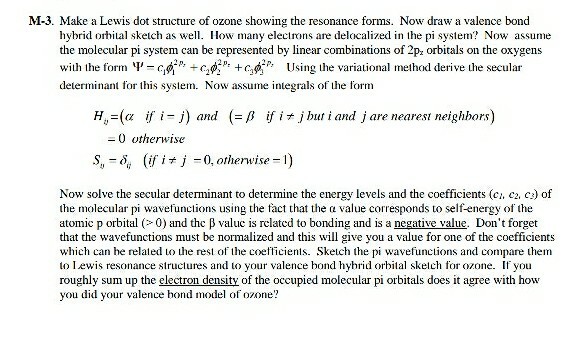
How Many Electrons Are Delocalized In The π System Of Ozone?
How many electrons are delocalized in the π system of ozone?. D How many electrons are delocalized in the π system of ozone. In ozone O3 the two oxygen atoms on the ends of the moleculeare equivalent to one another. View the full answer.
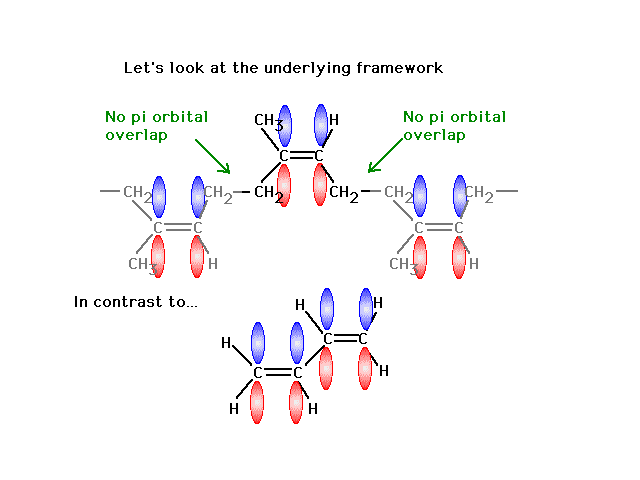
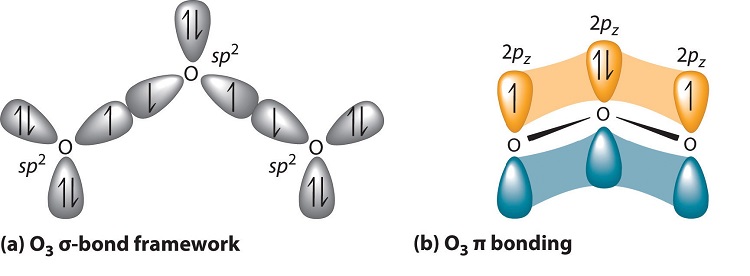
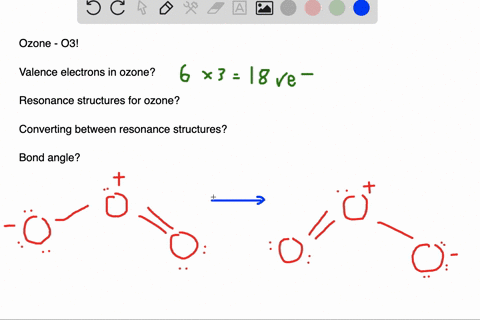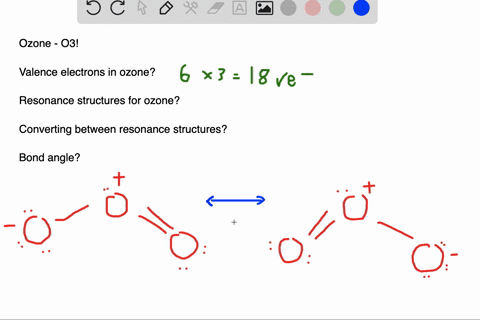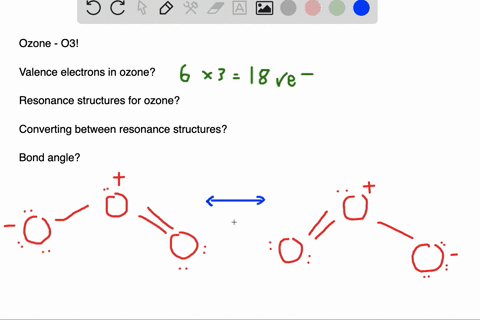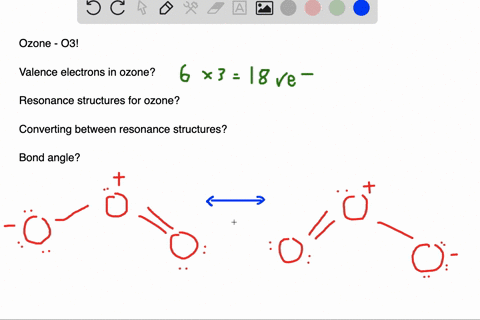
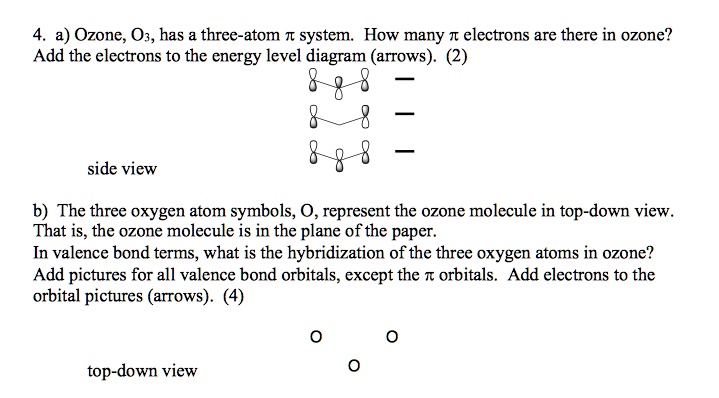
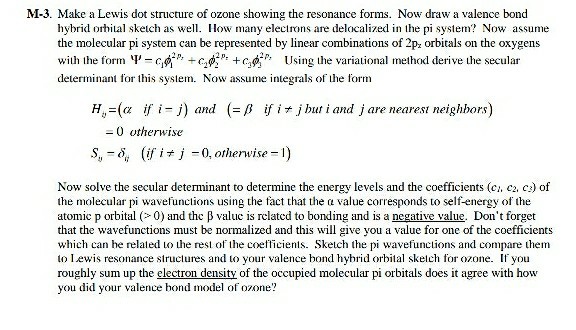
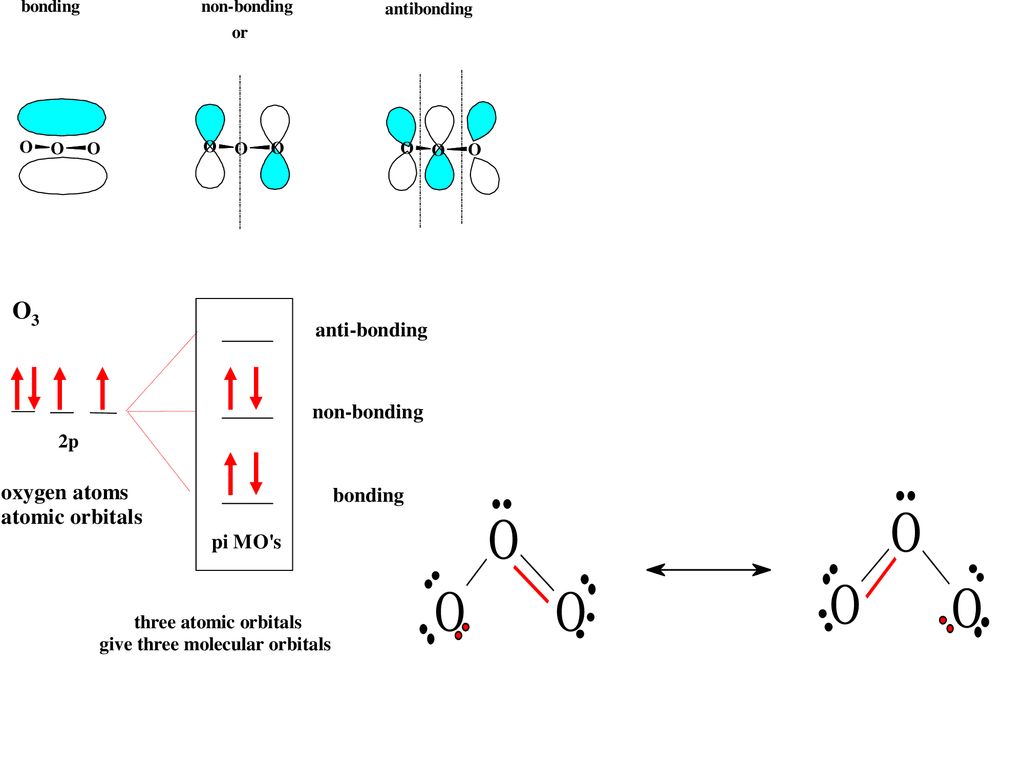
Delocalized bonds are when electron density is spread over more than two atoms for instance when 2p orbitals on the three oxygen atoms in ozone overlap to form a three-center bond in which two electrons are spread over the three atoms. Each oxygen atom in ozone has 6 valence electrons so O 3 has a total of 18 valence electrons. An electron shared by more than two atoms is said to be delocalized.
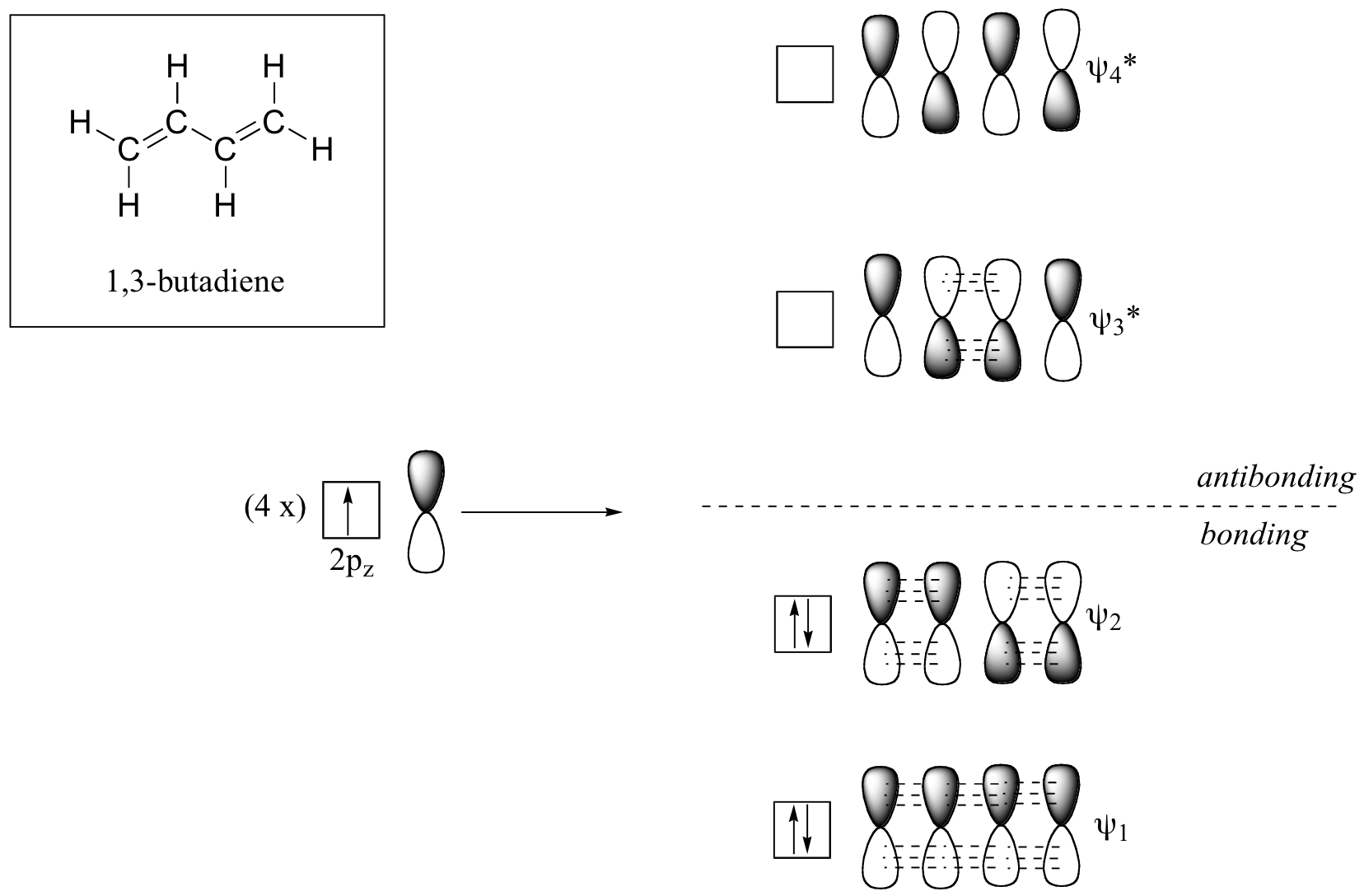
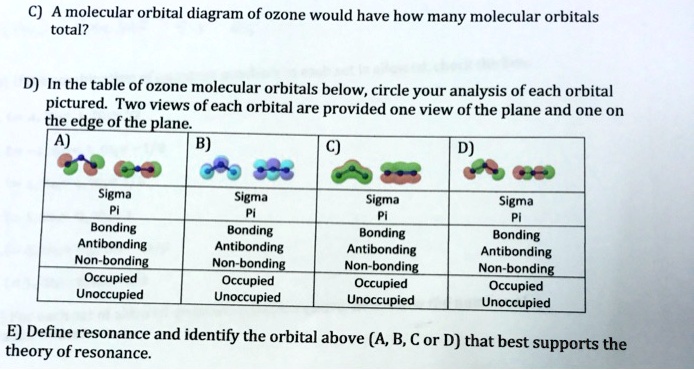
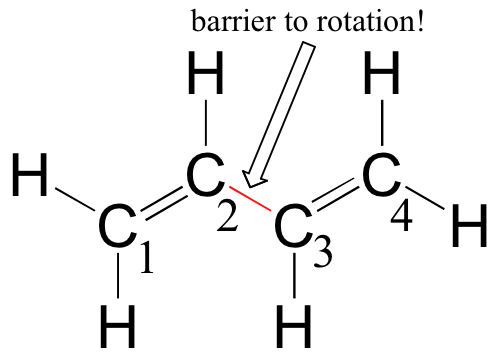
C Which of the orbitals can be used to delocalize the π electrons. This upsets examiners because a pi bond can only hold 2 electrons - whereas in benzene there are 6 delocalised electrons. The number of protons increases as the atomic number increases.
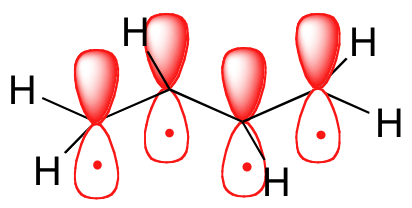
There are 4 electrons delocalized in the pi system. Furthermore each oxygen has a p orbital perpendicular to the plane of its sp2 hybrids that is perpendicular to the plane of the three oxygen atoms which has not yet been used for bonding. C The core electrons move closer to the.
In ozone O3 the two oxygen atoms on bartleby. 96 25 ratings answer - There are 4 electrons delocalized in the pi system. B For one of the resonance forms of ozone which of theorbitals are used to make bonds and which are used to.
We say that these π electrons are delocalized. Part A - Delocalized Bonding. We review their content and use your feedback to keep the quality high.
This involves only 14 of the 18 valence electrons of the three oxygens and so 4 electrons are left for pi bonding. The two pi electrons in the nitrate ion are shared by a total of four atoms one nitrogen atom and three oxygen atoms.
We say that these π electrons are delocalized.
Furthermore each oxygen has a p orbital perpendicular to the plane of its sp 2 hybrids that is perpendicular to the plane of the three oxygen atoms which has not yet been used for bonding. Experts are tested by Chegg as specialists in their subject area. D How many electrons are delocalized in the π system of ozone. It delves into molecular orbital theory. C Which of the orbitals can be used to delocalize the π electrons. This upsets examiners because a pi bond can only hold 2 electrons - whereas in benzene there are 6 delocalised electrons. Delocalized bonds are when electron density is spread over more than two atoms for instance when 2p orbitals on the three oxygen atoms in ozone overlap to form a three-center bond in which two electrons are spread over the three atoms. A What is the bestchoice of hybridization scheme for the atoms of ozone. How many electrons are in the π system of the ozone molecule o3.
Furthermore each oxygen has a p orbital perpendicular to the plane of its sp 2 hybrids that is perpendicular to the plane of the three oxygen atoms which has not yet been used for bonding. Experts are tested by Chegg as specialists in their subject area. The number of protons increases as the atomic number increases. Two occupy a molecular orbital containing node in the plane where the oxygen nuclei reside and the rest of the electron density is spread over the. In ozone O3 the two oxygen atoms on bartleby. B For one of the resonance forms of ozone which of the orbitals are used to make bonds and which are used to hold nonbonding pairs of electrons. We say that these π electrons are delocalized.



















Post a Comment for "How Many Electrons Are Delocalized In The π System Of Ozone?"